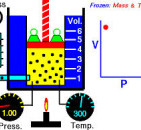
Boyle's law
States that when the temperature is held constant, the volume of a gas is inversely proportional to its pressure. Therefore, if the pressure increases, the volume decreases and visa versa. For example, if the volume if halved, then the pressure is doubled. If the temperature is held constant, it becomes an isothermal process. Discovered by Robert Boyle (1627-1691), an Irish physicist and chemist and co-founder of the Royal Society.
- قسم من أقسام الكلام: proper noun
- المجال / النطاق: البيئة الطبيعية
- الفئة: الغلاف الجوي
0
Other terms in this blossary
المنشئ
- Mona Liza
- 100% positive feedback
(Islamabad, Pakistan)